ChromaDex PURENERGY – Meeting Consumer Demand For Energy With Less Caffeine
Many consumers ingest caffeine on a daily basis in their coffee or tea — or even in colas and energy drinks — but the latest trend in the energy product sector is caffeine in food, and not just in the expected places, such as chocolate candy or coffee ice cream. Caffeine is now being added to foods such as oatmeal, waffles, potato chips, sunflower seeds, juices and marshmallows. Such foods are being marketed for their stimulant effects – i.e., an “action-packed” beef jerky juiced up with 75 milligrams of caffeine per serving or jelly beans with 50 milligrams of caffeine in each packet.
The addition of caffeine to a wider range of products and in larger amounts has raised concerns among lawmakers, regulators and consumers about the potential adverse health effects of excessive caffeine consumption.
The U.S. Congress, for instance, has been looking into adverse event reports of illness, injury and death linked to consumption of energy drinks. A congressional survey entitled “What’s All the Buzz About?” (http://www.markey.senate.gov/documents/04-10-13%20-%20Energy%20Drink%20report%20FINAL.pdf) cites a statistic that the number of emergency room visits linked to energy drinks doubled between 2007 and 2011 – from 10,0000 to 20,000. The congressional survey recommends the implementation of requirements for clear labeling on amounts of caffeine contained in energy products, precautionary statements for products with caffeine levels above those affirmed as safe by the FDA, the cessation of marketing to children and teens under 18 and increased reporting to the FDA of serious adverse events.
The FDA is also looking into regulating energy drinks and other energy products. In an Aug. 26 blog, FDA Deputy Commissioner Michael R. Taylor said: “By breaking far outside the traditional boundaries surrounding caffeine as a component of the food supply, these products pose challenging public health and regulatory questions. They stem from the fact that these products are being marketed explicitly for their stimulant properties, are prone to being consumed under a range of new and different conditions, and are providing caffeine in forms that are attractive and accessible to children and adolescents.” He went on to say that the FDA would take “whatever actions are needed” to protect the health of consumers. (http://blogs.fda.gov/fdavoice/index.php/2013/08/defining-boundaries-for-caffeine-in-todays-marketplace/
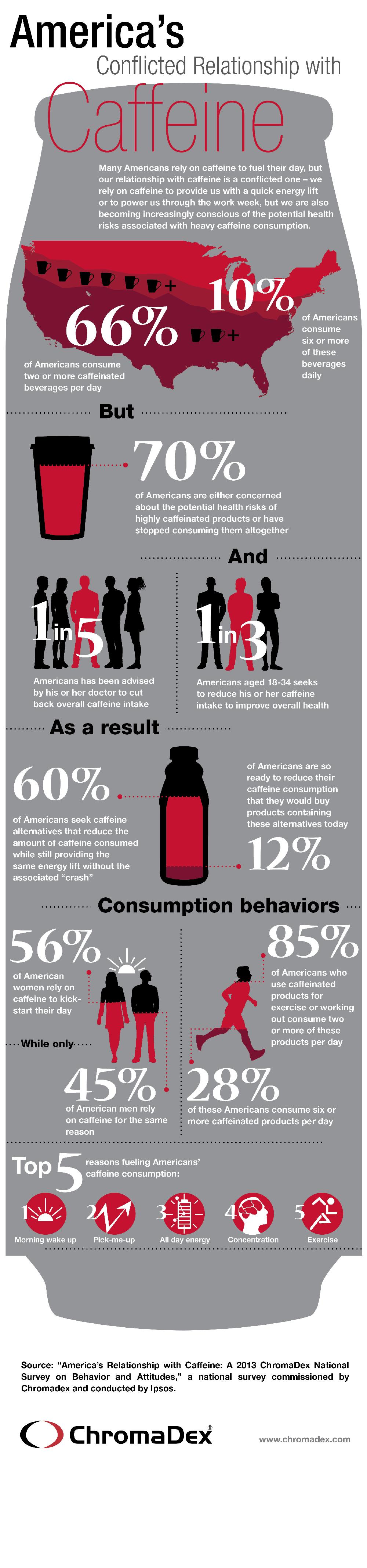
The increased consumption of caffeine has also alarmed consumers themselves. In a national survey on caffeine consumption conducted by ChromaDex, 70 percent of respondents reported either being concerned about the potential health risks of highly caffeinated products or having stopped consuming them altogether, with one in five having been advised by a health practitioner to cut back on caffeine. At the same time, the respondents expressed reluctance to give up their daily dose of caffeine, with 70 percent of those who said they wanted to reduce their caffeine intake to avoid negative side effects such as jitters, anxiety or increased heart rate still reporting that they consume two or more caffeinated beverages or products per day.
So how can the market respond to such competing demands: a concern about the negative effects of caffeine consumption combined with a reliance on caffeine to get through the day?
My company, ChromaDex, which specializes in developing ingredient solutions that answer specific needs in the marketplace, has developed a caffeine alternative called, PURENERGY, a patented co-crystal consisting of caffeine and pTeroPure, the branded nature-identical form of pterostilbene, which is a natural ingredient found in blueberries. In a human clinical study conducted by researchers from the University of Mississippi, pTeroPure demonstrated that it has calming properties, including the ability to lower blood pressure and reduce anxiety (http://hyper.ahajournals.org/cgi/content/meeting_abstract/60/3_MeetingAbstracts/A617). Since the negative effects of caffeine are hypertension and anxiety related, we believe that by combining caffeine and pTeroPure, the resulting compound could mitigate the negative effects of caffeine without affecting consumers’ experience. The results of our first human clinical evaluation of PURENERGY, which were recently presented at 34th Annual Meeting of the American College of Toxicology in San Antonio, confirmed its ability to deliver longer sustained energy at reduced dosages and without the “crash.”
These results suggest that PURENERGY can be an alternative to caffeine for formulators of energy products, as it has the ability to reduce levels of caffeine by as much as 50 percent without sacrificing consumer expectations. The human clinical evaluation also showed how PURENERGY achieves these results: the rate of caffeine absorption is significantly slower with PURENERGY — by about 30 percent as compared to ordinary caffeine — and the half-life (the time required for half of the original amount to leave the body) of the caffeine in PURENERGY is extended significantly, by about 25 percent over that of ordinary caffeine. Also, at six hours compared to baseline, PURENERGY showed improvement in focus, energy, concentration, fatigue and alertness. Caffeine did not. In other words, PURENERGY can offer the same energy lift as traditional caffeine in significantly lower quantities, which will allow energy product formulators to meet any restrictions on caffeine levels that may be mandated by federal regulators in the future.
ChromaDex’s national survey of caffeine consumption habits also indicated that there is strong consumer demand for an ingredient such as PURENERGY: 60 percent of respondents said they would be willing to consume a reduced caffeine beverage if it provided the same benefits and feel without the negative side effects and “crash.” The enormous consumer demand for energy products, confirmed by the results of our consumer survey, indicates that consumers are very reluctant to give up their caffeine boost. But if Congress or the FDA does crack down on caffeine levels, the market will have an alternative in the form of a caffeine product that delivers the goods with less caffeine.
For more information on PURENERGY™, please visit the “Ingredients” section of the ChromaDex website at www.chromadex.com.
About Author
Frank L. Jaksch Jr. is the founder and chief executive of ChromaDex, an innovative natural products company that provides proprietary, science-based solutions and ingredients to the dietary supplement, food and beverage, animal health, cosmetic and pharmaceutical industries.